Which Member of Each Pair Is More Soluble in Water
H2 Ar CH2Cl2 NH3 H2S CH3OH HCN PF5 HF BrCl5. CH3CHOl is more soluble than H2Ol.

Entry Task Nov 19th Monday Ppt Download
Which member of each pair is more soluble in water.

. I hexan-2-ol or ii cyclooctane-14-diol c. CH a or b or c or Br d CHOH e f or or Br. SrSO4 or MgSO4 b.
A A CH3CH2OCH3 g or B CH3CH2OCH2CH3 l b A CH2Cl2 l or B CCl4 l c A tetrahydropyran or B cyclohexane. Okay which for reference is ch three c h 20 ch two ch three. Classify the species given below by the intermolecular forces present in a pure sample of each.
They dont cancel each other out. A Hexan-1-ol or cyclohexanol. So out of these three pairs all of the ones on the left would be soluble in water.
So for part C were given slightly bigger molecules. Answer to Circle the member of each pair that is more soluble in water. For part a Would you see that the first compound that just contains chlorine xyz non polar while the other one is polar thats the polar molecule will be more likely soluble in water.
We can observe this by. Ammonia is soluble in water. So this is a six member carbon ring all carbon carbon single bonds.
Which member of each pair is more soluble in water CH3CH2OH OR CH3CH2OCH3. Pair be ch three c n to ch three. Solution for Predict which member of each pair is more soluble in water.
Chemistry questions and answers. Glucose can form hydrogen bond with water because of presence of -OH group. Siege three ch three.
Predict which member of each pair will be more soluble in water. Predict which member of each pair will be more soluble in water. Present in all molecules.
CH3CH2OH is more soluble in water. The alcohol and water can exhibit hydrogen bonding while the ether cannot. Explain the reasons for your answers.
Which member if each pair is likely to be more soluble in water a. CH3CH2OH is more soluble in water because it is polar the alcoholic OH group is polar whereas the CH3CH2OCH3 is not polar so not so soluble in water. A weak acid is an acid that is partially dissociated into its ions in an aqueous solution or water.
1 Answer to Which member of each pair is more soluble in water. Oh thisll one here on the left would be soluble in water. Uh first we have cyclo vexing.
We work on problem 13 from chapter 13 in this problem were asked to decide which of each of several players would be more soluble in the solvent di ethyl ether. Double O or siege three. So the forward reaction will not favorable.
Circle the member of each pair that is more soluble in watera CH3CH2OCH2CH3 or CH3CH2CH2CH2CH3b CH3CH2OCH2CH3 or CH3CH2CH2OHc CH3CH2NHCH3 or CH3CH2CH2CH3d CH3CH2OH or CH3CH2CH2CH2OH. We have two compounds that exhibit hydrogen bonding one with a very short non polar chain and. And so that means that we have uh and um and effective die poll.
Cyclohexane is non-polar molecule and can interact with water only through. Step 1 of 5. CH3CH2MgBrs is more soluble than MgBr2s.
B Heptan-1-ol or 4-methylphenol. Explain in each case. Which of the following in each pair is likely to be more soluble in water.
Among the ethyl methyl ether and diethyl ether the ethyl methyl ether is having smaller size when compared to the diethyl ether hence the solubility of is more in water compared to the solubility of in water. I heptan-1-ol or ii 4-methylphenol b. CH3CH2OCH3g CH3CH2OCH2CH3l CH2Cl2l CCl4l Tetrahydropyran Cyclohexane In recent years the country with the least number of bankruptcies was.
Which member of each pair is more soluble in water. CNN would be soluble in water pair. A CH3CH2OCH2CH3 or CH3CH2CH2CH2CH3 b CH3CH2OCH2CH3 or CH3CH2CH2OH c CH3CH2NHCH3 or CH3CH2CH2 SolutionInn.
Cast of this question it is helpful to draw the chemical structures although you do not have to. A Both methylethylether and diethyl ether consists of an oxygen atom that engages in hydrogen bonding with water molecules. - I think it worth adding that the ether is a gas at room temperature.
Name three interactions that stabilize the shape of a soluble globular protein and identify the structural features that allows for these interactions. Fifty-five solubility data points were found collected from 29 studies. So dyke Laura methane would be the one thats more soluble in water.

Amongst The Following Compounds Identify Which Are Insoluble Partially Soluble And Highly Soluble In Water I Phenol Ii Toluene Iii Formic Acid Iv Ethylene Glycol V Chloroform Vi Pentanol

Acrylic Markers Set Of 40 Paint Pens For Wood Paper Metal Glass Video Video Painting Art Projects Acrylic Markers Marker Art
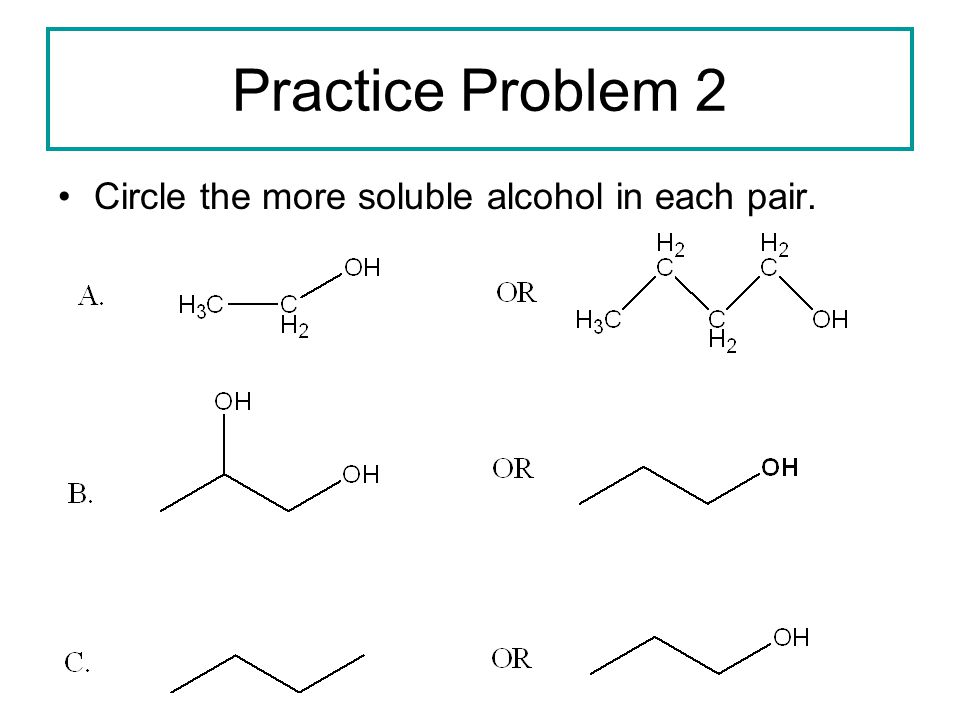
Alcohols Phenols Thiols And Ethers Ppt Video Online Download

Chapter 11 Liquids Solids And Intermolecular Forces Ppt Download

Covalent Bonds Covalent Chemical Bonds Involve The Sharing Of A Pair Of Valence Electrons By Two At Teaching Chemistry Science Chemistry Chemistry Education
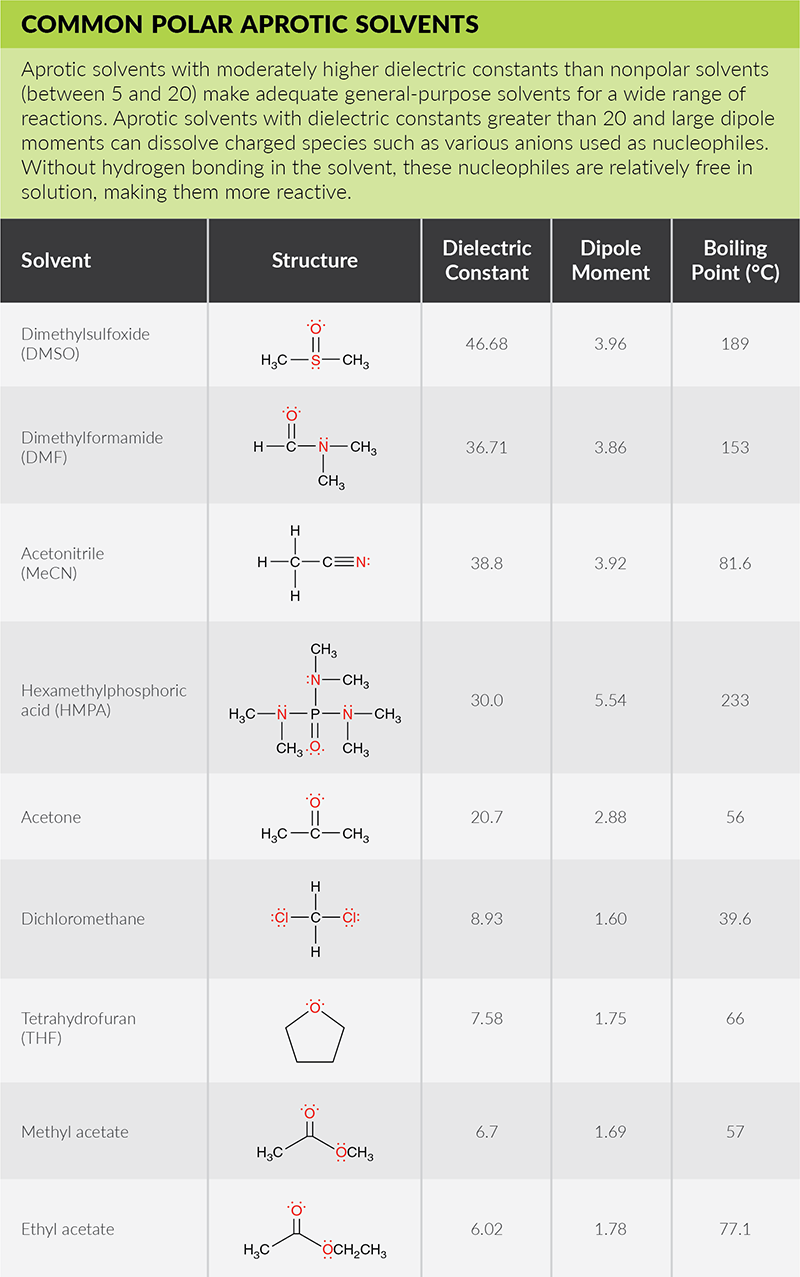
Solubility Factors When Choosing A Solvent News Announcements Cayman Chemical

Sidekick Sling Bag Sewing Pattern By Betz White Bag Betz Pattern Sewing Sidekick Sli Bag Patterns To Sew Sewing Patterns Sling Bag

Mack S Pillow Soft Silicone Earplugs 2 Pack 2 Pairs Each Walmart Com Swimmers Ear Soft Silicone Earplugs

0 Response to "Which Member of Each Pair Is More Soluble in Water"
Post a Comment